The _____ Functional Group Can Always Be Found in a Carbohydrate Molecule
Carbohydrates can comprise hydroxyl (alcohol) groups, ethers, aldehydes and/or ketones.
Carbohydrates are chains (or polymers) of bones sugar molecules such equally glucose, fructose and galactose. In order to come across which functional groups are present in carbohydrates, we must look at the functional groups present in the more basic building blocks. Saccharides - and by extension carbohydrates - are composed of simply three atoms: carbon, hydrogen and oxygen. The structure for one of the most common saccharides, glucose, is shown here.
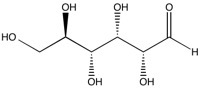
Here we can identify multiple hydroxyl (alcohol) functional groups and one aldehyde functional group. Alcohols are characterized past
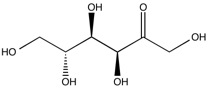
Here, because the
Lastly, we must consider functional groups that ascend through the linking of saccharides. Below is the structure of a disaccharide sugar consisting of glucose and fructose. Notice that here both glucose and fructose are fatigued in their cyclic ring form.
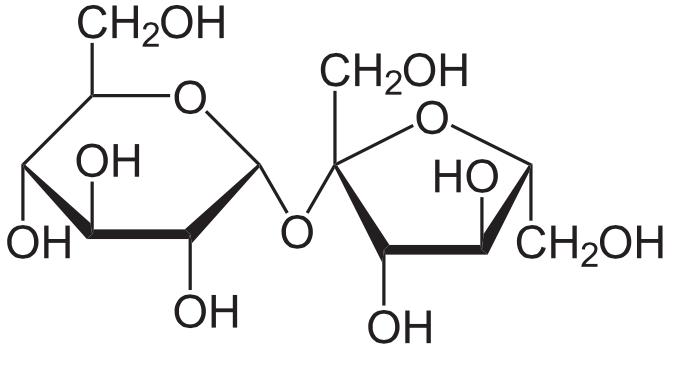
The two saccharides are linked through an oxygen atom. This link is chosen a glycosidic bond. Since the glycosidic bond has the form,
Source: https://socratic.org/questions/what-functional-groups-are-present-in-carbohydrates
0 Response to "The _____ Functional Group Can Always Be Found in a Carbohydrate Molecule"
Post a Comment